

You've lost 16 grams of stuff, and in this reaction that just doesn't happen! Where did the 16 grams go? What this says is that you start with 34 grams of stuff and end up with 18 grams of stuff. If you do some of the gram molecular weight calculations you will find this:Ģ grams of hydrogen + 32 grams of oxygen = 18 grams of water We can form water by combing hydrogen gas (H2) and oxygen (O2) in the presence of electricity.

In other words, if I start baking bread with 10 pounds of flour, I should end up with 10 pounds of bread, unless some is lost onto the floor or if some of it goes up in smoke!Ī simple example goes a long way. This is a fundamental skill in chemistry, as you might have noticed from the short reading in stoichiometry! Balancing equations means writing chemical equations such that the amount of stuff you start with in the reaction equals the amount of stuff you end up with as a product. Acids, Bases & Salts and It's Properties.Daniell Cell with Construction and Working:.Metals and Non-Metals with Physical and Chemical.
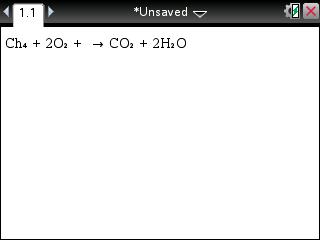
Law of Conservation of Mass and Landolt Experiment.Definitation of Alpha Particles, Beta Particles an.Empirical Formula and Molecular Formula:.Percent Composition, Empirical Formula,Molecular W.


 0 kommentar(er)
0 kommentar(er)
